Laparoscopic intra-ovarian platelet rich plasma injection for ovarian rejuvenation: a new hope for infertile women
Mohamed Farag El Sherbeny*
Department of Obstetrics and Gynecology, Faculty of Medicine, Benha University, Egypt
Received: 04 September 2020
Revised: 20 September 2020
Accepted: 21 September 2020
*Correspondence:
Dr. Mohamed Farag El Sherbeny,
E-mail: mohamedsherbeny2020@gmail.com
Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
This case report was presented to show the outcome of bilateral laparoscopic platelet rich plasma intra-ovarian injection for 34-year old woman who had primary infertility secondary to premature ovarian insufficiency and had two previously failed attempts of ICSI. the procedure, One month after the patient had menses and her hormonal profile was improved with increased serum AMH and E2 and decreased serum FSH and LH. On the eleventh day of the menstrual cycle, folliculometry detected good follicle measuring 18×20 mm, on the fifteenth day, trans-vaginal ultrasonography assured ovulation and timed intercourse was ordered, and she got pregnant. At the thirty fifth gestational week, she had premature preterm rupture of membrane and urgent cesarean delivery was performed and the newborn was admitted to neonatal intensive care unit. As conclusion, intra-ovarian PRP injections could be safe, productive, and a natural treatment for women with POI. Laparoscopic injection allowed perfect under-vision intra- ovarian injection and can be conducted as one-day procedure and this can be considered a novelty of the applied procedure
INTRODUCTION
Premature ovarian insufficiency (POI) is the cessation of menstruation before age of forty yearsand affects 1-3% of adult women.1,2 Etiopathogenesis of POI is theoretically divergent, but may be related to cancer chemo and/or radiotherapy.3 Presence of short telomeres and diminished telomerase activity in granulosa cells and also carriers of BRCA1 and BRCA2 genes mutations are at higher risk of POI.4,5
Unfortunately, till now, no therapeutic intervention was found to effectively restore fertility in women with POI and hormonal manipulations to stimulate ovarian function were proved unsuccessful.2 Platelet rich plasma (PRP) is an autologous highly concentrated product of the whole blood that was derived by gradient density centrifugation to contain a number of platelets in a small plasma volume.6 PRP is an autologous source of platelet derived growth factor and transforming growth factor-β (TGF-β) that are obtained by sequestering and concentrating platelets by gradient density centrifugation.7 PRP is an inexpensive source of many growth factors in physiological proportion, so it was widely used as a therapyand was proved effective for tissue repair and regeneration.7,8
CASE REPORT
A 34 year old woman presented with oligomenorrhea with little menstrual flow for only two days and 3 year primary infertility despite normal husband’s seminogram with high percentage of viable and forward motile sperms. She had two previous trials of ICSI but unfortunately resulted in only one follicle and failed to give an embryo. The second trial gave three follicles but none of it was suitable for ICSI and the trial was cancelled.
The patient had had a breast lump that was proved pathologically to be benign after excision. Patient was clinically evaluated and gave blood samples for determination of baseline hormonal profile (Table 1). She was asked to refrain hormonal or other medications for at least one month before committing the trial.
PRP was prepared using the Ycellbio™ PRP kits (Ycellbio Medical Company Ltd., USA) according to the manufacturer’s instructions. Fourteen ml of peripheral blood were obtained under complete aseptic conditions and were added to 1 ml sodium citrate as anticoagulant. The tubes were centrifuged at 3,500 rpm for 5 minutes at room temperature and 10 ml of PRP were withdrawn in two 5 ml syringes.
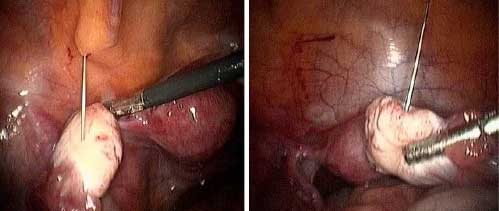
The injection procedure was performed under intravenous anesthesia with sedation, using conventional system through two 1.5-2 cm incisions. Briefly, the abdomen was insufflated with CO2 to a maximum pressure of 14-16 mmHg according to requirement. After abdominal exploration, the needle was advanced into the right ovary and 5 ml of PRP was injected into the ovarian stroma and then, the left ovary was injected using the other 5 ml of PRP (Figure 1). After assuring absence of needle insertion site bleeding, the apparatus was removed and wound was closed. Patient was managed postoperatively as day case and was discharged after resumption of independent motility and receiving oral fluid. Unprotected intercourse was advised and patient was asked to attend the hospital one-month after the procedure or if menstruation occurred or its predicted data was missed.
Table 1: Patient’s hormonal profile estimated at first clinical attendance and on third day of menstrual cycle at one month after procedure.
| Hormones | Baseline | One month after procedure |
| Serum anti-Müllerian hormone (ng/ml) | 0.01 | 0.29 |
| E2 (pg/ml) | 19 | 52 |
| Serum follicle stimulating hormone (mIU/ml) | 18.3 | 10.2 |
| Serum leutinizing hormone (mIU/ml) | 9.3 | 6 |
| Serum prolactin (ng/ml) | 7.5 | 8.7 |
| Serum thyroid stimulating hormone (µIU/ml) | 0.52 | 0.49 |
| Serum thyroxin (µg/dl) | 6.7 | 7.9 |
| Serum tri-iodotyrosine (ng/dl) | 118 | 134 |
Figure 1: Bilateral intraovarian PRP laparoscopic injection.
One month after the procedure, the patient had menses and, on the third menstrual day hormonal profile was re- determined. Fortunately, serum AMH level was increased by 29 folds and serum E2 was increased by 3 folds in comparison to pre-procedure levels. Meanwhile, serum FSH and LH levels were decreased to about 55% and 65% of pre-procedure levels, respectively. Also, serum T3, T4 and prolactin levels were increased, while serum TSH level was decreased, but the change was un- meaningful (Table 1). On the 11th day of the menstrual cycle, folliculometry detected good follicle measuring 18×20 mm and on the 15th day, TUV detected ovulation.
Timed intercourse was ordered in trial to get the highest chance for getting pregnant.
On the 2nd month after the procedure, fortunately, the patient had missed period and pregnancy test was positive and TVU detected a fetal sac on the 20th day. Fortunately, the patient completed her pregnancy uneventfully till the 35th gestational week whenever, she had premature preterm rupture of membrane and urgent cesarean delivery was conducted and the newborn was admitted to neonatal intensive care unit.
DISCUSSION
Premature ovarian insufficiency/failure is considered as a heterogeneous disease state with different underlying pathogenic mechanisms, so individualized therapeutic strategy for each patient is advocated. The currently applied therapeutic strategy provided multiple advantages, on biochemical scale intra-ovarian PRP injection allowed shift of patient form POI to ovarian activity status as manifested by the increased serum AMH and E2 by 29 and 3 folds, respectively in conjunction with decreased serum FSH and LH by 55 and 65%, respectively. On physiological scale, our patient had two active cycles, the first results in menstruation and the second allowed getting pregnant.
These results go in hand with Sills et al who using US- guided transvaginal PRP injection for four women with diminished ovarian reserve who had at least one prior canceled IVF cycle because of poor follicular recruitment response, had reported increased serum AMH and/or decreases in FSH levels in all cases and these changes allowed retrieval of about 5 MII oocytes and IVF occurred after about 80 days after PRP injection.9 Also, Sfakianoudis et al reported a natural IVF cycle that led to a biochemical pregnancy after PRP but resulted in a spontaneous abortion at the 5th gestational week and Pantos et al detected improvement in hormonal profile of POI women with decreased FSH levels and a concurrent increase of AMH levels following PRP treatment.10,11 Thereafter, Sfakianoudis et al out of their pilot study including women with POI, poor ovarian response, perimenopause and menopause, reported significant improvement on the hormonal profile and the ovarian reserve status with intra-ovarian PRP infusion and detected a menstruation recovery rate of 60% of women had POI or poor ovarian response, 40% of menopausal women positively responded to PRP treatment and 80% of perimenopausal women had menstruation regularity.12
Our presented case was allowed to get unprotected intercourse after intra-ovarian PRP injection till detection of a mature follicle and subsequent ovulation on the second month, timed intercourse was undertaken and resulted in chemical and clinical pregnancy. These data indicated the feasibility of getting natural pregnancy and thus the applied procedure spared the need for pregnancy assisted techniques with its inherent failure rate and high cost especially with this patient who had previous two unsuccessful trials of ICSI.
In line with these findings, Cakiroglu et al reported that after PRP injection, 7.4% of studied women conceived spontaneously and 26.4% attempted IVF and developed embryos. Also, Petryk tried intra-ovarian PRP injection in 38 women with low ovarian reserves and had at least two unsuccessful attempts to receive oocytes through IVF and detected significant improvement in hormone levels, ten women got pregnant, four of them from natural conception and six healthy babies were born. Melo et al reported overall rates of biochemical (26.1% versus 5.4%) and clinical pregnancy (23.9% versus 5.4%) in women with abnormal ovarian reserve markers received monthly intracortical ovarian PRP injections for three cycles versus no intervention.13,14,15
These favorable outcomes for PRP could be attributed to its content of multiple growth factors which may promote ovarian neovascularization with concomitant increased intra-ovarian blood supply as previously suggested by Sfakianoudis et al who attributed the effect of PRP to its content of essential factors for neoangiogenesis.10 Another attribute was the improved local ovarian immune milieu secondary to content of PRP of anti-inflammatory cytokines that may antagonize the local inflammatory process that may be responsible for follicular atresia.16 Moreover, the anti-apoptotic activity of platelet contents may allow stoppage of apoptotic process accused to be the underlying pathogenic mechanism for POI.17
In support of these assumptions, Vural et al using POI- animal model found co-transplantation of MSCs and PRP allowed increased expression of TGF-β and insulin-like growth factor-1 concomitantly with increased expression of CXCL12 which functions as an anti-inflammatory chemokine.18 Thereafter, Ahmadian et al using POI- animal model found PRP injection reduced the number and extent of the follicular atresia and inflammatory responses.19 Moreover, PRP was found to suppress gene expression of angiopoietin 2 (ANGPT2) which is encoded protein that disrupts the vascular remodelling ability of ANGPT1 and may induce endothelial cell apoptosis, and up-regulate the expression of tyrosine- protein kinase that acts as a cell-surface receptor for vascular endothelial growth factor, thus plays an essential role in the regulation of angiogenesis, vascular development and permeability.20
CONCLUSION
Intra-ovarian PRP injections could be safe, productive, and a natural treatment for women with POI especially those with previously failed attempts of assisted reproduction techniques. Laparoscopic injection allowed perfect under-vision intra-ovarian injection and can be conducted as one-day procedure and this can be considered a novelty of the applied procedure.
Funding: No funding sources
Conflict of interest: None declared
Ethical approval: Not required
REFERENCES
- Di-Battista A, Moysés-Oliveira M, Melaragno Genetics of premature ovarian insufficiency and the association with X-autosome translocations. Reproduction. 2020;160(4):R55-64.
- Igboeli P, El Andaloussi S, Sheikh U, Takala H, ElSharoud A, McHugh A, et Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature. J Med Case Rep. 2020;14(1):108.
- Dolmans M, Donnez J. Fertility preservation in women for medical and social reasons: Oocytes vs ovarian Best Pract Res Clin Obstet Gynaecol. 2020;S1521-6934(20):30122-X.
- Fattet A, Toupance S, Thornton S, Monnin N, Guéant J, Benetos A, et Telomere length in granulosa cells and leukocytes: a potential marker of female fertility? A systematic review of the literature. J Ovarian Res. 2020;13(1):96.
- Merlino L, Chiné A, Galli C, Piccioni M. BRCA1/2 genes mutations, ovarian reserve and female reproductive outcomes: a systematic literature Minerva Ginecol. 2020;72(3):64-70.
- Kumar K, Rao J, Kumar B, Mohan A, Patil K, Parimala K . A prospective study involving the use of platelet rich plasma in enhancing the uptake of bone grafts in the oral and maxillofacial region. J Maxillofac Oral Surg. 2013;12(4):387-94.
- Schwartz-Arad D. Reconstraction of severe anterior maxillary ridge atrophy: combination of several surgical Refuat Hapeh Vehashi- nayim. 2017;34(1):45-51.
- Pennati A, Apfelbeck T, Brounts S, Galipeau J. Washed equine platelet extract (WEPLEX) as an anti-inflammatory biologic Tissue Eng Part A. 2020;26(15-16):902-10.
- Sills E, Rickers N, Li X, Palermo First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol. 2018;34(9):756-60.
- Sfakianoudis K, Simopoulou M, Nitsos N, Rapani A, Pappas A, Pantou A, et al. Autologous platelet-rich plasma treatment enables pregnancy for a woman in premature menopause. J Clin 2019;8(1):1.
- Pantos K, Simopoulou M, Pantou A, Rapani A, Tsioulou P, Nitsos N, et al. A case series on natural conceptions resulting in ongoing pregnancies in menopausal and prematurely menopausal women following platelet-rich plasma Cell Transplant. 2019;28(9-10):1333-40.
- Sfakianoudis K, Simopoulou M, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, et Reactivating ovarian function through autologous platelet-rich plasma intraovarian infusion: pilot data on premature ovarian insufficiency, perimenopausal, menopausal, and poor responder women. J Clin Med. 2020;9(6):1809.
- Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk S, Scott R, et Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging. 2020;12(11):10211-22.
- Petryk N, Petryk Ovarian rejuvenation through platelet-rich autologous plasma (prp)-a chance to have a baby without donor eggs, improving the life quality of women suffering from early menopause without synthetic hormonal treatment. Reprod Sci. 2020;27(7):70-8.
- Melo P, Navarro C, Jones C, Coward K, Coleman L. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non- randomized interventional J Assist Reprod Genet. 2020;37(4):855-63.
- Elkady M, Shalaby S, Fathi F, El-Mandouh Effects of quercetin and rosuvastatin each alone or in combination on cyclophosphamide-induced premature ovarian failure in female albino mice. Hum Exp Toxicol. 2019;38(11):1283-95.
- Yefimova M, Lefevre C, Bashamboo A, Eozenou C, Burel A, Lavault M, et al. Granulosa cells provide elimination of apoptotic oocytes through unconventional autophagy-assisted Hum Reprod. 2020;35(6):1346-62.
- Vural B, Duruksu G, Vural F, Gorguc M, Karaoz E. Effects of VEGF + mesenchymal stem cells and platelet-rich plasma on inbred rat ovarian functions in cyclophosphamide-induced premature ovarian insufficiency Stem Cell Rev Rep. 2019;15(4):558-73.
- Ahmadian S, Sheshpari S, Pazhang M, Bedate A, Beheshti R, Abbasi M, et Intra-ovarian injection of platelet-rich plasma into ovarian tissue promoted rejuvenation in the rat model of premature ovarian insufficiency and restored ovulation rate via angiogenesis modulation. Reprod Biol Endocrinol. 2020;18(1):78.
- Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound J Dent Res. 2005;84(5):434-9.
Cite this article as: El Sherbeny MF. Laparoscopic intra-ovarian platelet rich plasma injection for ovarian rejuvenation: a new hope for infertile women. Int J Reprod Contracept Obstet Gynecol 2020;9:4320-3.